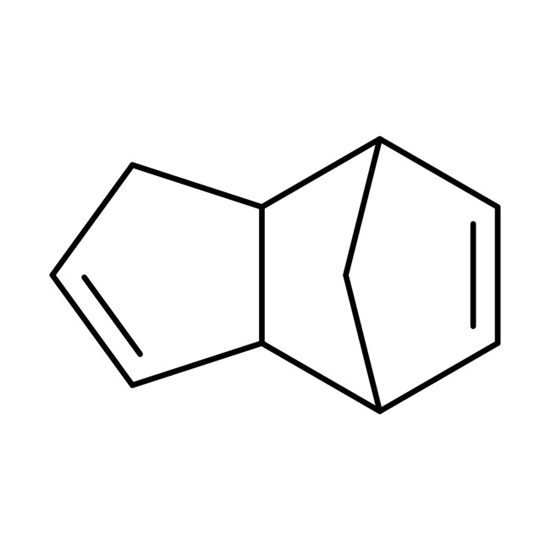
Dicyclopentadiene (DCPC for short) is a dimer of cyclopentadiene. First heating is used to copolymerize cyclopentadiene into dicyclopentadiene, dicyclopentadiene Chemicalbook is separated from other light components (boiling point <45°C) by distillation, and then other required dienes, Monoolefin and saturated hydrocarbon components. High-purity dicyclopentadiene is a colorless crystal at room temperature. When it contains impurities, it is a light yellow oily liquid with a pungent camphor smell. It is insoluble in water and soluble in organic solvents such as alcohol and ether.
Distill the light benzene fraction of coal tar, cut out the benzene head fraction below 70°C, which contains about 30% cyclopentadiene, then heat, polymerize, and distill to obtain the finished product dicyclopentadiene. It is obtained from the C5 fraction in the hydrocarbon cracking industry as the raw material, which is heated and dimerized, and then separated by distillation under reduced pressure. Secondly, it can also be obtained by dimerization with cyclopentadiene as raw material. Process flow:

1. Using the C5 fraction as the raw material, firstly heat the C5 fraction to 80-120°C to dimerize the cyclopentadiene. Then, rectification is performed, and a dicyclopentadiene fraction is obtained by separation from the column bottom. Because in addition to cyclopentadiene, isoprene, etc., this fraction also contains diolefins. When it is heated to above 170℃, only dicyclopentadiene is preferentially decomposed and rectified. The obtained cyclopentadiene is thermally polymerized and rectified to obtain dicyclopentadiene.
2. Cyclopentadiene can be converted into dimer with a boiling point of 170°C by thermal polymerization, and then purified by distillation. The dimerization reaction was most complete at 70°C but the reaction process was very slow. Use heating under total reflux for 16-18h, and then heat up to take the front fraction (below 82°C) and the benzene fraction (above 82°C) until the kettle temperature reaches 110°C. The residue at the bottom of the kettle is dicyclopentadiene with a content of about 60%, and then through vacuum distillation, a dimer with a content of ≥99% can be obtained.
Clent Chemical is a company that sells dicyclopentadiene, our dicyclopentadiene is of good quality. If you want to learn more about dicyclopentadiene, please stay tuned to our website. We will regularly update relevant articles and products, and provide relevant product information and materials, and look forward to your visit and consultation.