Introduction to Antimicrobial Susceptibility
Antimicrobial susceptibility testing, also known as antibiogram or drug sensitivity testing, is a laboratory technique used to determine the sensitivity of bacteria, viruses, fungi or parasites to antimicrobial drugs such as antibiotics, antivirals, antifungals and antiparasitics. This helps clinicians determine the most appropriate therapy for infections caused by these microorganisms. The tests are performed either by measuring the zone of inhibition or minimum inhibitory concentration of a drug against the microbial isolate.
Methods of Antimicrobial Susceptibility Testing
There are a few main methods used for antimicrobial susceptibility testing in clinical microbiology laboratories:
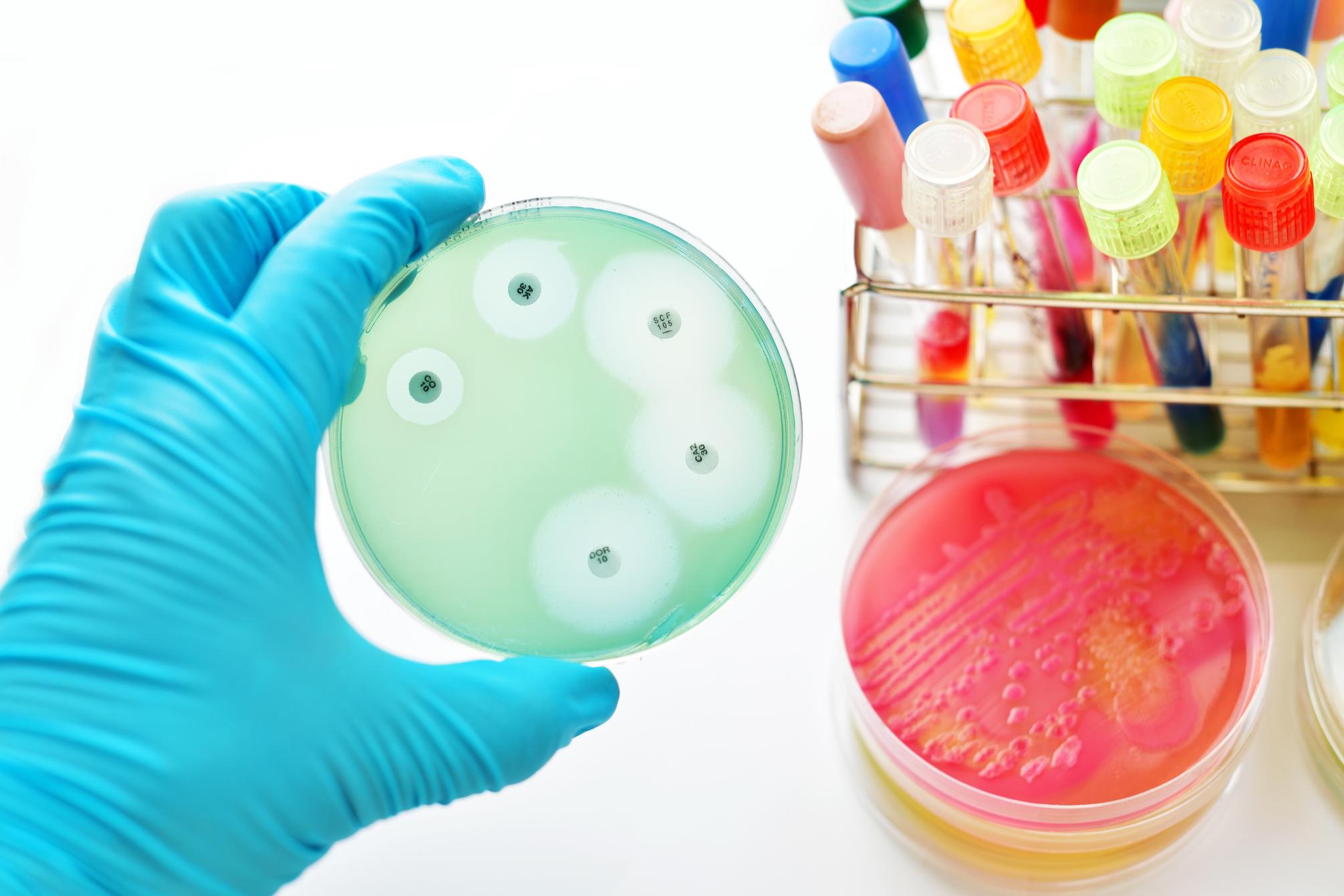
Disk Diffusion Test: This test determines susceptibility or resistance of a microbial isolate by measuring the diameter of the zone of inhibition around Antimicrobial Susceptibility Testing discs on an inoculated agar plate. A larger zone of inhibition indicates that the microorganism is more susceptible to that particular drug.
Broth Dilution Test: In this test, serial dilutions of an antimicrobial agent are prepared in broth and a fixed volume of a standardized microbial inoculum is added. The lowest concentration of the antimicrobial drug that prevents visible growth is determined as the minimum inhibitory concentration or MIC.
Automated Systems: Automated systems expedite the testing process and standardize interpretation of results. They use colorimetric or turbidimetric methods to determine MIC values within 8-24 hours for routine testing of bacteria against a panel of drugs.
Etest: This uses plastic agar-backed strips impregnated with a continuous gradient of antimicrobial drug concentrations. After incubation, MIC is read directly from the strip where growth meets the ellipse-shaped inhibition zone.
Reporting and Interpretation of Results
The results of antimicrobial susceptibility tests are interpreted and reported as susceptible, intermediate or resistant based on predefined clinical breakpoints established by regulatory bodies such as CLSI or EUCAST. Susceptible indicates the antimicrobial is likely to be effective for treatment, resistant means therapy with that agent should be avoided, while intermediate susceptibility is uncertain. Factors affecting breakpoints include pharmacokinetics/pharmacodynamics of drugs and MIC distributions for pathogen populations.
Get more insights on Antimicrobial Susceptibility Testing