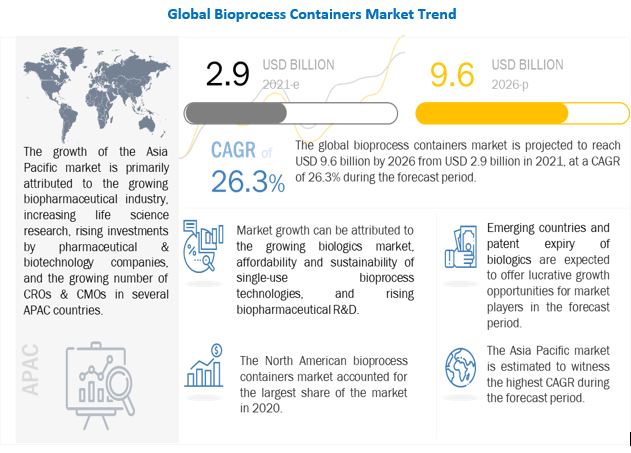
The global bioprocess containers market is on track to reach a staggering $9.6 billion by 2026, showcasing remarkable growth potential in the biopharmaceutical industry. This projection is backed by crucial industry insights that shed light on the factors driving this exponential growth.
Download PDF Brochure-https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=107645832
Growing Biologics Market Fuels Demand
One of the key drivers propelling the bioprocess containers market is the substantial growth in the biologics market. Biologics, which encompass a range of therapeutic products derived from living organisms, have gained immense popularity in recent years due to their efficacy and potential for personalized medicine. The production of biologics demands high sterility, making single-use bioprocess technologies a preferred choice. These technologies offer better, cheaper, and faster production methods, aligning with the industry's increasing need for efficient biologics production.
The COVID-19 pandemic further accelerated the demand for biologics, particularly vaccines. This surge in demand led to a notable increase in the usage of single-use bioreactors, bioprocess containers, and related consumables such as media bags and filtration assemblies.
Additionally, the approval of 53 new medicines and therapeutic biologics by the US FDA in 2020 signifies the substantial growth in the biologics market. Monoclonal antibodies and human insulin, among other biologics, have been pivotal contributors to the bioprocess containers market's expansion.
Leachables and Extractables Pose Challenges
While single-use bioprocess technologies offer numerous advantages, including reduced process validation requirements and enhanced manufacturing flexibility, they also present challenges. One of the significant concerns is the potential for leaching of organic compounds or inorganic substances from the plastic materials used in single-use systems. This undesirable phenomenon could compromise the safety, quality, and purity of the final drug product.
Extractables, compounds that can be extracted from source materials under rigorous laboratory conditions, and leachables, compounds present in drug products due to container leaching, are common issues associated with polymeric and elastomeric materials. Concerns regarding these substances may hinder the widespread adoption of single-use bioprocess containers in the future.
Opportunities Arise with Patent Expiry of Biologics
As patents for several biologic products expire, companies are reallocating resources from research and development (R&D) to manufacturing. This shift has led to a growing emphasis on cost-effective production methods. Consequently, biopharmaceutical manufacturers are increasingly incorporating single-use bioprocessing technologies into their development processes. By utilizing single-use bioprocessing assemblies like bioprocess containers, companies can create innovative products within budget constraints.
Waste Disposal Presents Challenges
One of the challenges faced by biomanufacturing companies utilizing single-use assemblies is the disposal of solid waste. Single-use containers often comprise multiple materials, making it difficult to separate components for recycling. This generates substantial volumes of solid waste, increasing waste management costs and posing an environmental challenge.
Download PDF Brochure-https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=107645832
In conclusion, the bioprocess containers market is poised for significant growth, driven by the expanding biologics market and the cost-effectiveness of single-use bioprocess technologies. However, concerns related to leachables and extractables and waste disposal must be addressed to ensure sustainable growth in this sector. As biopharmaceutical companies continue to innovate and adapt to changing market dynamics, the future of bioprocess containers remains promising.


